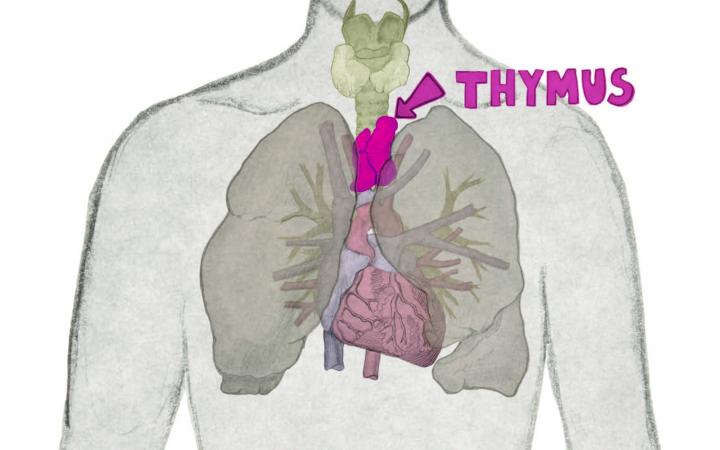
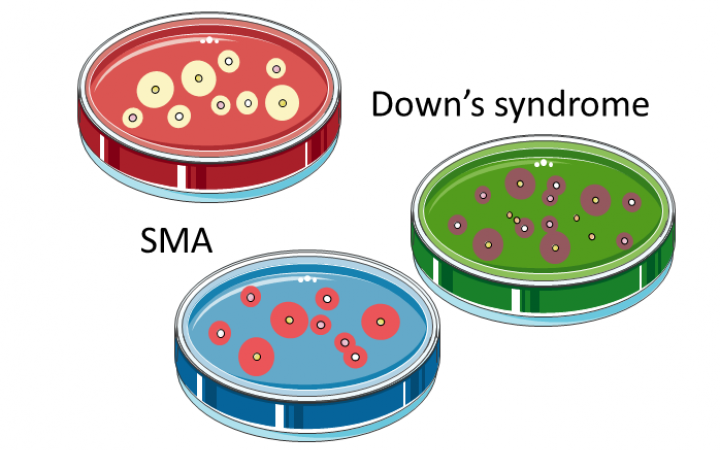
Esplora le Cellule Staminali
In questa sezione forniamo un'introduzione alle cellule staminali, ai loro utilizzi, alla ricerca, alle schede informative, alle politiche in materia, alle news ed altri argomenti collegati.
Se non sai bene da dove iniziare, ti consigliamo di dare una occhiata alle schede informative.