Explore Stem Cells
Want to know more about stem cells and their uses? In this section, we provide an introduction to stem cells and their uses, research, factsheets, policy, news and other stem cell themes.
If you are not sure where to start, we recommend browsing our Factsheets.
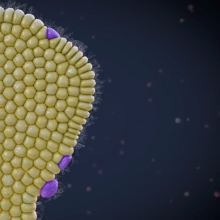
Video: The Birth of Beta Cells
• EuroStemCell resource
Sporty Stem Cells: altitude training and stem cells
• EuroStemCell resource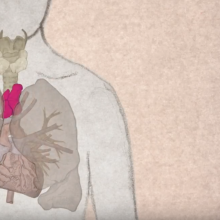
Video: Inside the Thymus
• EuroStemCell resource
Sporty Stem Cells: how stem cells can help cartilage injury
• EuroStemCell resource
Sporty Stem Cells: how stem cells can help tendon damage
• EuroStemCell resource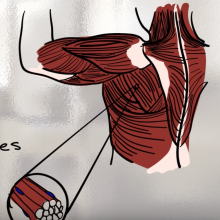
Sporty Stem Cells: how stem cells make muscle
• EuroStemCell resource
Short film: Unfolding Collaboration
• EuroStemCell resource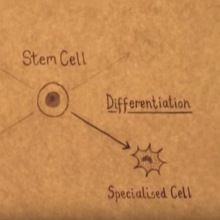
What is cloning, and what does it have to do with stem cell research?
• EuroStemCell resource
Hope Beyond Hype
• EuroStemCell resource
Dolly and Beyond
• EuroStemCell resource
Cell Culture
• EuroStemCell resource
Conversations: Ethics, Science, Stem Cells
• EuroStemCell resource
Stem cells - the Future: an introduction to iPS cells
• EuroStemCell resource
Cell Fate: Journeys to Specialisation
• EuroStemCell resource
A Stem Cell Story
• EuroStemCell resource
Diabetes: how could stem cells help?
• EuroStemCell resource
Cord blood stem cells: current uses and future challenges
• EuroStemCell resource
Embryonic stem cell research: an ethical dilemma
• EuroStemCell resource
