Cord blood stem cells: current uses and future challenges
Umbilical cord blood was once discarded as waste material but is now known to be a useful source of blood stem cells. Cord blood has been used to treat children with certain blood diseases since 1989 and research on using it to treat adults is making progress. So what are the current challenges for cord blood research and how may it be used – now and in the future?
Cord blood is contained in the umbilical cord and placenta of a newborn child. It can be easily collected and frozen for later use.
Cord blood contains blood (haematopoietic) stem cells, which can produce all the other cells found in blood, including cells of the immune system.
Transplants of haematopoietic stem cells (HSCs) from cord blood can be used to treat several different blood diseases, such as leukaemia.
Compared to HSCs from bone marrow donors, transplants of HSCs from cord blood appear to lead to fewer immune system incompatibilities, such as graft-versus-host disease.
A limitation of cord blood is that it contains fewer HSCs than a bone marrow donation does, meaning adult patients often require two volumes of cord blood for treatments. Researchers are studying ways to expand the number of HSCs from cord blood in labs so that a single cord blood donation could supply enough cells for one or more HSC transplants.
Some controversial studies suggest that cord blood can help treat diseases other than blood diseases, but often these results cannot be reproduced. Researchers are actively investigating if cord blood might be used to treat various other diseases.
A large challenge facing many areas of medical research and treatments is correcting misinformation. Some companies advertise services to parents suggesting they should pay to freeze their child’s cord blood in a blood bank in case it’s needed later in life. Studies show it is highly unlikely that the cord blood will ever be used for their child. However, clinicians strongly support donating cord blood to public blood banks. This greatly helps increase the supply of cord blood to people who need it.
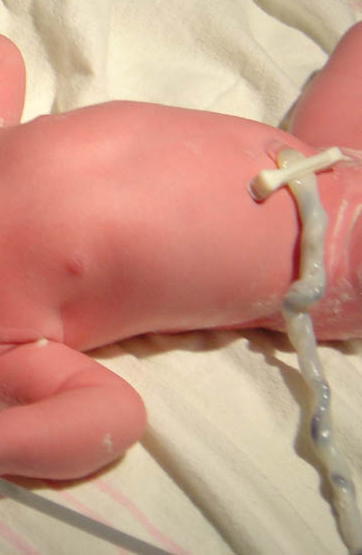
After a baby is born, cord blood is left in the umbilical cord and placenta. It is relatively easy to collect, with no risk to the mother or baby. It contains haematopoietic (blood) stem cells: rare cells normally found in the bone marrow.
Haematopoietic stem cells (HSCs) can make every type of cell in the blood – red cells, white cells and platelets. They are responsible for maintaining blood production throughout our lives. They have been used for many years in bone marrow transplants to treat blood diseases.
There have been several reports suggesting that cord blood may contain other types of stem cells which can produce specialised cells that do not belong to the blood, such as nerve cells. These findings are highly controversial among scientists and are not widely accepted.
Cord blood is used to treat children with cancerous blood disorders such as leukaemia, or genetic blood diseases like Fanconi anaemia. The cord blood is transplanted into the patient, where the HSCs can make new, healthy blood cells to replace those damaged by the patient’s disease or by a medical treatment such as chemotherapy for cancer.
In this way, cord blood offers a useful alternative to bone marrow transplants for some patients. It is easier to collect than bone marrow and can be stored frozen until it is needed. It also seems to be less likely than bone marrow to cause immune rejection or complications such as Graft versus Host Disease. This means that cord blood does not need to be as perfectly matched to the patient as bone marrow (though some matching is still necessary).
However, cord blood transplants also have limitations. Treatment of adults with cord blood typically requires two units of cord blood to treat one adult. Clinical trials using "double cord blood transplantation" for adults have demonstrated outcomes similar to use of other sources of HSCs, such as bone marrow or mobilized peripheral blood. Current studies are being done to expand a single cord blood unit for use in adults. Cord blood can also only be used to treat blood diseases. No therapies for non-blood-related diseases have yet been developed using HSCs from either cord blood or adult bone marrow.
A major limitation of cord blood transplantation is that the blood obtained from a single umbilical cord does not contain as many haematopoeitic stem cells as a bone marrow donation. Scientists believe this is the main reason that treating adult patients with cord blood is so difficult: adults are larger and need more HSCs than children. A transplant containing too few HSCs may fail or could lead to slow formation of new blood in the body in the early days after transplantation. This serious complication has been partially overcome by transplanting blood from two umbilical cords into larger children and adults. Results of clinical trials into double cord blood transplants (in place of bone marrow transplants) have shown the technique to be very successful. Some researchers have also tried to increase the total number of HSCs obtained from each umbilical cord by collecting additional blood from the placenta.
Much research is focused on trying to increase the number of HSCs that can be obtained from one cord blood sample by growing and multiplying the cells in the laboratory. This is known as “ex vivo expansion”. Several preliminary clinical trials using this technique are underway. The results so far are mixed: some results suggest that ex vivo expansion reduces the time taken for new blood cells to appear in the body after transplantation; however, adult patients still appear to need blood from two umbilical cords. More research is needed to understand whether there is a real benefit for patients, and this approach has yet to be approved for routine clinical use.
Several research teams have reported studies in animals suggesting that cord blood can repair tissues other than blood, in diseases ranging from heart attacks to strokes. These findings are controversial: scientists often cannot reproduce such results and it is not clear HOW cord blood may be having such effects. When beneficial effects are observed they may be very slight and not significant enough to be useful for developing treatments. If there are positive effects, they might be explained not by cord blood cells making nerve or heart cells, but by the cells in the cord blood releasing substances that help the body repair damage.
Current research aims to answer these questions in order to establish whether safe and effective treatments for non-blood diseases could be developed in the future using cord blood. An early clinical trial investigating cord blood treatment of childhood type 1 diabetes was unsuccessful. Other very early stage clinical trials are now exploring the use of cord blood transplants to treat children with brain disorders such as cerebral palsy or traumatic brain injury. However, such trials have not yet shown any positive effects and most scientists believe much more laboratory research is needed to understand how cord blood cells behave and whether they may be useful in these kinds of treatments
Experts believe that umbilical cord blood is an important source of blood stem cells and expect that its full potential for treatment of blood disorders is yet to be revealed. Other types of stem cell such as induced pluripotent stem cells may prove to be better suited to treating non-blood-related diseases, but this question can only be answered by further research.
As the research into umbilical cord blood and it’s therapeutic use for blood diseases has grown, so has the question as to whether people should privately store the cord blood of their offspring for future use. A recent paper on this issue by Mahendra Rao and colleagues advocates the practice of cord blood banking (for treatment of blood diseases) but in the context of public cord blood banks rather than a private cord blood banks. Any adult needing treated would need at least two cord blood samples that are immune compatible. So one sample will not be sufficient. A child might only need one cord blood sample but in the case of childhood leukaemia there is a risk that pre-leukemic cells are present in cord blood sample - and so the child could not use their own cells for therapy.
If everyone donated cord blood to public registries for the 'common good' this would increase the chances of someone benefiting from a double cord blood transplant. This far outweights the actual probability of the person who donated the sample being able to usefully use it for themself.
A review article on this issue from 2008 summarises the public versus private cord blood banks debate comprehensively and concludes:
“This reanalysis supports several previously expressed opinions that autologous [to use one's OWN cells] banking of cord blood privately as a biological insurance for the treatment of life-threatening diseases in children and young adults is not clinically justified because the chances of ever using it are remote. The absence of published peer-reviewed evidence raises the serious ethical concern of a failure to inform prospective parents about the lack of future benefit for autologous cord banking … Attempts to justify this [commercial cord blood banking] are based on the success of unrelated public domain cord banking and allogeneic [using someone ELSE'S cells] cord blood transplantation, and not on the use of autologous [the person’s OWN cells] cord transplantation, the efficacy of which remains unproven”.
Umbilical cord blood is useful for research. For example, researchers are investigating ways to grow and multiply haematopoietic (blood) stem cells from cord blood so that they can be used in more types of treatments and for adult patients as well as children. Cord blood can also be donated altruistically for clinical use. Since 1989, umbilical cord blood transplants have been used to treat children who suffer from leukaemia, anaemias and other blood diseases.
There are over 130 public cord blood banks in 35 countries. They are regulated by Governments and adhere to internationally agreed standards regarding safety, sample quality and ethical issues. In the UK, several NHS facilities within the National Blood Service harvest and store altruistically donated umbilical cord blood. Trained staff, working separately from those providing care to the mother and newborn child, collect the cord blood. The mother may consent to donate the blood for research and/or clinical use and the cord blood bank will make the blood available for use as appropriate.
Cord blood in public banks is available to unrelated patients who need haematopoietic stem cell transplants. Some banks, such as the NHS bank in the UK, also collect and store umbilical cord blood from children born into families affected by or at risk of a disease for which haematopoietic stem cell transplants may be necessary - either for the child, a sibling or a family member. It is also possible to pay to store cord blood in a private bank for use by your own family only.
Cord blood can be stored in public or private (commercial) cord blood banks.
For example, in the UK the NHS Cord Blood Bank has been collecting and banking altruistically donated umbilical cord blood since 1996. The cord blood in public banks like this is stored indefinitely for possible transplant, and is available for any patient that needs this special tissue type. There is no charge to the donor but the blood is not stored specifically for that person or their family.
Companies throughout Europe also offer commercial (private) banking of umbilical cord blood. A baby's cord blood is stored in case they or a family member develop a condition that could be treated by a cord blood transplant. Typically, companies charge an upfront collection fee plus an annual storage fee.
There has been considerable debate about the ethical and practical implications of commercial versus public banking. The main arguments against commercial banking have to do with questions about how likely it is that the cord blood will be used by an individual child, a sibling or a family member; the existence of several well-established alternatives to cord blood transplantation and the lack of scientific evidence that cord blood may be used to treat non-blood diseases (such as diabetes and Parkinson’s disease). In some cases patients may not be able to receive their own cord blood, as the cells may already contain the genetic changes that predispose them to disease.
Umbilical cord blood contains haematopoietic (blood) stem cells. These cells are able to make the different types of cell in the blood - red blood cells, white blood cells and platelets. Haematopoietic stem cells, purified from bone marrow or blood, have long been used in stem cell treatments for leukaemia, blood and bone marrow disorders, cancer (when chemotherapy is used) and immune deficiencies.
Since 1989, umbilical cord blood has been used successfully to treat children with leukaemia, anaemias and other blood diseases. Researchers are now looking at ways of increasing the number of haematopoietic stem cells that can be obtained from cord blood, so that they can be used to treat adults routinely too.
Beyond these blood-related disorders, the therapeutic potential of umbilical cord blood stem cells is unclear. No therapies for non-blood-related diseases have yet been developed using HSCs from either cord blood or adult bone marrow. There have been several reports suggesting that umbilical cord blood contains other types of stem cells that are able to produce cells from other tissues, such as nerve cells. Some other reports claim that umbilical cord blood contains embryonic stem cell-like cells. However, these findings are highly controversial among scientists and are not widely accepted.
EuroStemCell fact sheet on blood stem cells (HSCs)
International Society for Stem Cell Research: Cord Blood & Uses to Treat Disease
The European Group for Blood and Marrow Transplantation
UK National Health Service cord blood bank
Umbilical Cord Blood Banking - an opinion paper by the Royal College of Obstetricians and Gynaecologists’ Scientific Advisory Committee (published June 2006)
This factsheet was created by Rajeev Gupta and reviewed by Tariq Enver, with additional advice from Alexander Medvinsky. Reviewed in 2016 by Dan S Kaufman. Edited by Emma Kemp and Jan Barfoot.
Lead image of baby's umbilical cord from Wikimedia Commons. Possible human blood stem cell image by Rajeev Gupta and George Chennell. Remaining images of blood sample bags and red blood cells from Wellcome Images.