Lung stem cells in health, repair and disease
Lung diseases like chronic obstructive pulmonary disease (COPD), cancer, lung fibrosis and pneumonia are among the most common lung diseases in Europe. These diseases can cause severe, protracted illness and ultimately death, meaning long hospitalisation times, suffering and high costs. Research on lung stem cells helps to understand the onset of these diseases and is helping to develop treatments with the goal of reducing suffering and costs.
Many chronic lung diseases, such as COPD and fibrosis, are caused by changes in cells that make up the lung. Important cell populations in the lung are the stem cell populations. In healthy people the job of the lung stem cells is to maintain the normal lung structure. When they receive specific cues, stem cells function by dividing to replace old, or damaged, more specialist lung cells. In chronic lung diseases this process can go wrong leading to long-term breathing difficulties and ultimately death.
Pneumonia is another lung disease with a very high death rate, particularly among the elderly. Pneumonia is usually triggered by a lung infection and it can be caused by flu or COVID-19. Severe pneumonia is treated with mechanical ventilation which itself can cause lung damage. The lungs should be able to repair damage caused by ventilation by activating their stem cells to make new healthy lung tissue. However, when the damage is too severe the repair process cannot happen normally and fibrosis (when healthy cells are replaced by scar tissue) can occur.
A better understanding of how stem cells in the lung can help to repair damage, and of how stem cell behaviour is altered in chronic lung diseases and severe infections, is crucial for finding new treatments.
Most of the research focused on lung stem cells is performed in either mice or human tissue. We can isolate lung stem cells from donated human tissue and grow them into ‘organoid cultures’ which resemble mini-organs. This method allows researchers to alter the instructions received by the stem cells and observe what happens. Researchers can, for example, compare healthy stem cells with stem cells from patients. Organoid cultures can also be used as a drug screening model, and even serve as a basis for patient precision medicine. In that way stem cells may one day be used to cure lung diseases.
In a different type of research, modern genetic tools can be used for “correction” of disease-causing unhealthy genes. A good example of this is the correction of unhealthy versions of the CFTR gene in Cystic Fibrosis. So far these gene corrections have only been performed on cells growing in the laboratory, but scientists are actively working on ways to make the same changes in patient’s lungs.
Researchers can use human stem cells in organoid cultures, but a large challenge is that this is only a model and the cultures only recapitulate some aspects of human lung biology.
Much of the lung stem cell research that has been done so far has been performed in mice. Recent studies in cell profiling of both mice and human samples have revealed many new cell types that are present in the lungs. The function of all these different cell types is not known so far and using mice is an excellent way for researchers to study the functions of these new cell types in an intact lung. Mouse and human lungs are constructed in a very similar way, however, it has also become clearer that there are significant differences in cell populations between mouse lungs and human lungs. Another difference between mouse and human is how ageing occurs. This is important as many severe lung diseases mostly affect older people.
To work alongside the experiments on human cells in a dish, researchers are increasingly trying to find additional animal models that more closely resemble humans. A model in between mouse and human that could be used is pig. Pig physiology and anatomy resembles the human lung and body much better than that of mice.
Our lungs work hard. In an average lifetime, human lungs take 20-40 million breaths and experience a daily airflow of between 7000 and 10,000 litres. Mammalian lungs are made up of two distinct regions:
1. The conducting airway tubes, including the trachea, bronchi, and bronchioles.
2. The gas exchange regions, or alveolar spaces.
Scientists have discovered that these regions each contain unique types of stem cells. In normal lungs, many stem cells are present throughout each region. These cells divide to replace old or damaged lung cells, which keeps the lung healthy. The stem cells include tracheal basal cells, bronchiolar secretory cells (known as club cells), and alveolar type 2 cells. Division of these stem cells is thought to be sufficient to renew the lung's structure throughout normal adult life.
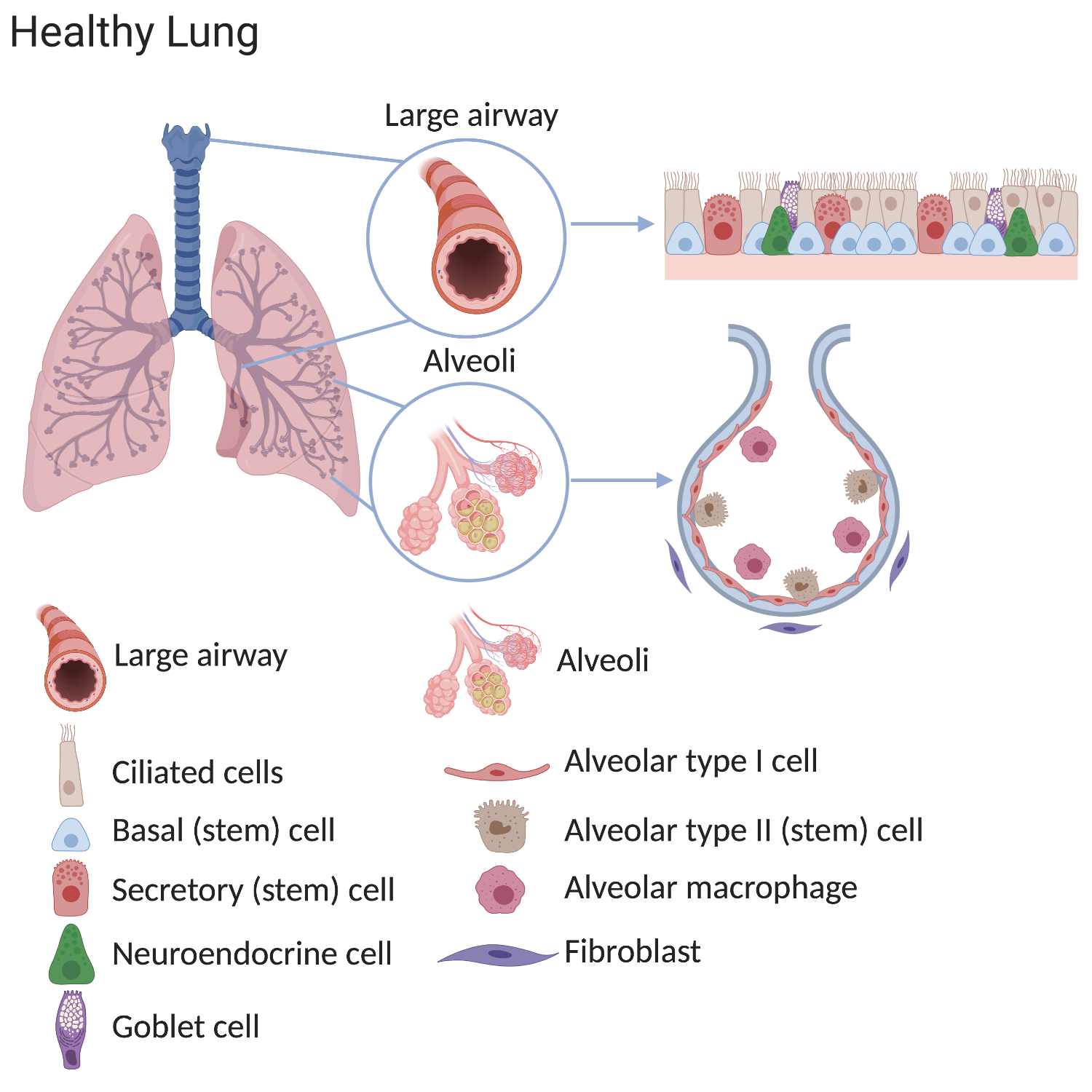
In lung diseases these processes are altered and therefore fewer functional cells are produced after damage. This means that the lung’s ability to supply the patient’s body with oxygen decreases over time causing illness and, eventually, death.
For example, the lungs of patients with chronic lung diseases such as COPD show alterations in the airways and in the alveoli, especially after exposure to cigarette smoke. The large airways of COPD sufferers show basal cell hyperplasia (an increased number of basal cells), loss of ciliated cells and an increased number of mucous-secreting goblet cells. In the alveoli of COPD lungs there are less alveolar type I cells. In healthy lungs alveolar type II cells can make type I cells and although this process still happens in COPD lungs it is no longer efficient. The environment surrounding the stem cell also changes in disease. There are more activated macrophages and fibroblasts found in alveoli of COPD lungs and these surrounding cells can provide signals which alter stem cell behaviour.
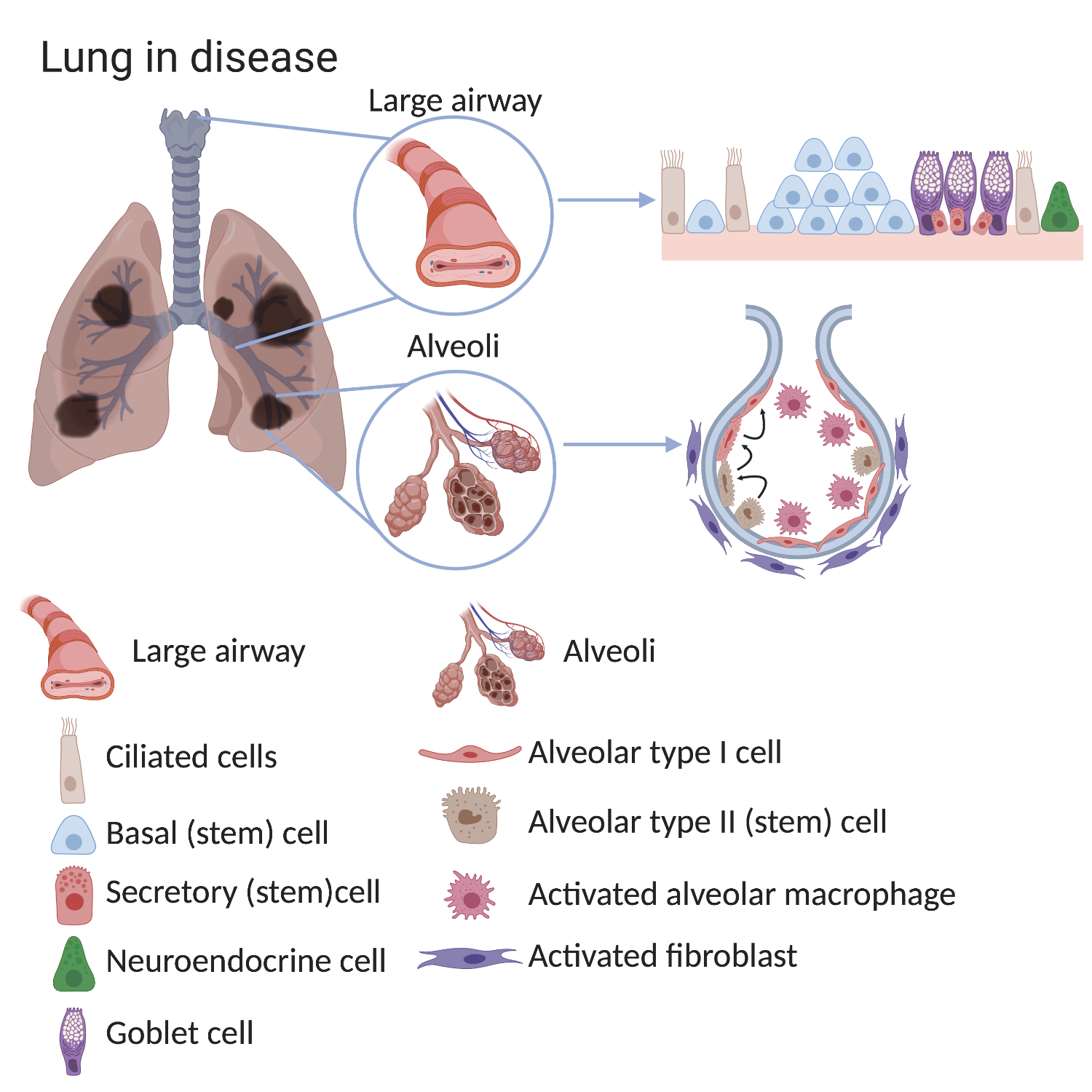
Some stem cells contribute to initial lung development and others help to repair and regenerate the lung throughout life. Problems with stem cells can contribute to lung diseases. In mouse lungs, rare stem cells have been discovered that are located in the conducting airway tubes. In response to severe injury (e.g. flu infection) these rare stem cells can divide and produce new cells that contribute to both the airway and gas exchange regions. These cells have also been grown in the lab and used as a treatment in injured mouse lungs. However, it is not yet clear if these rare stem cells are important in human lungs.
A better understanding of lung stem cell biology will improve our knowledge of how the lung works. We can do that by growing lung stem cells in a dish where they maintain their stem cell identity. By adding different behavioural cues found in healthy and diseased lungs to the dish, we can influence stem cell growth and mimic certain aspects of disease. When specific cues which can alter stem cell behaviour are identified in a dish in the laboratory, they can then be tested in animal models of disease. In this way, an improved understanding of how stem cells grow and respond to damage can help to further develop ways to manipulate the stem cells in the body and to find better treatment for diseases.
Current research is mainly focused on how we can indefinitely grow the different stem cells of the lung. This often happens in organoid (mini-organ) cultures which are particularly good for growing cells in the lab that behave like cells in the body. These cultures are being used for disease modelling and drug screening to attempt to identify new treatments for disease.
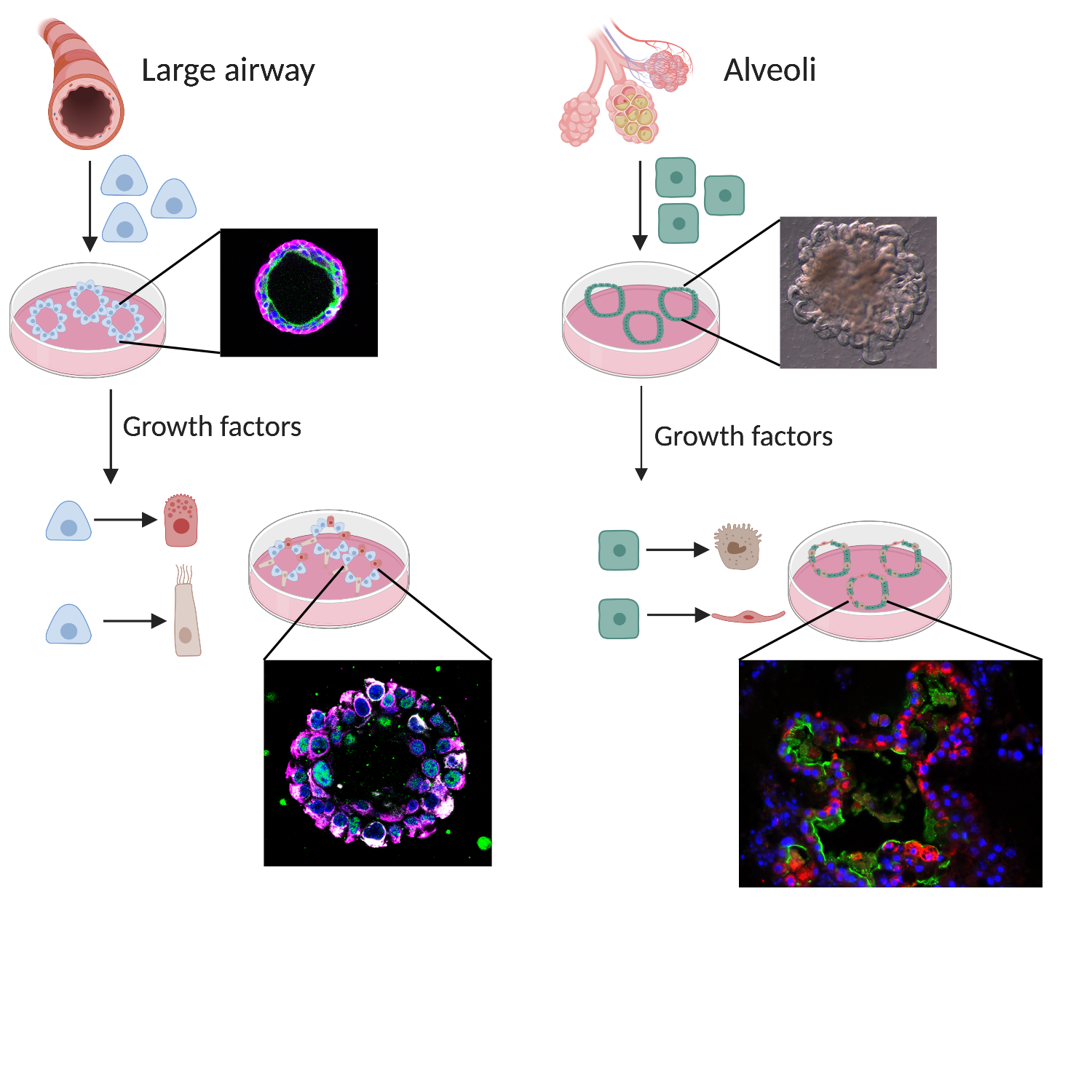
Animal experiments are still necessary for testing new drugs in the context of the whole lung and the whole body.
Donated patient material is used for organoid cultures to further identify differences between healthy and diseased lungs in response to certain stem cell behaviour cues and how we can manipulate this. Many labs around the world are trying to improve the methods for growing lung cancers as organoids. This would allow similar experiments – manipulating stem cell behaviour cues or drug screening – to be performed on lung cancers.
New methods are being developed to “gene correct” (repair damaged genes) in human cells. These methods work well in the lab and one day could lead to a new type of personalised medicine which may cure people whose genes cause them to develop certain lung diseases. A great deal of research is currently being carried out into how to safely alter the DNA of a patient, or how to safely give patients new lung stem cells with altered DNA.
As scientists improve their knowledge about human lung stem cells and use innovative new research methods, the path to clinical applications and new treatments will become shorter.
Great improvement has been shown in correcting genes in Cystic Fibrosis patient stem cells. Because of the ability to very precisely alter genes, this technique will be of great importance in the future for curing diseases which are caused by the individual’s DNA.
European Lung Foundation - public information in multiple European languages
British Lung Foundation - information and helpline for patients
European Respiratory Society - professional organisation for lung specialists
Scientific review article on airway basal stem cells
Scientific review article on lung stem cells and their contribution to disease
This factsheet was created in 2020 by Heleen Kool and Emma Rawlins.
It is a new version of the original factsheet created by Adam Giangreco and Emma Rawlins in 2010, then reviewed and updated in 2015 and 2018 by Emma Rawlins.
Diagrams by Heleen Kool. Images by Marko Nikolić and Kyungtae Lim.