COVID-19: Can we close the doors of our cells to Coronavirus SARS-CoV-2?
Scientists are examining whether targeting the way coronavirus enters our cells could be a pathway towards treatment. Sílvia Llonch summarises a paper from an international collaboration, including researchers from EuroStemCell's partner IMBA – Institute of Molecular Biotechnology of the Austrian Academy of Sciences.
- SARS-CoV-2: Severe Acute Respiratory Syndrome CoronaVirus 2. This virus is responsible for the disease COVID-19.
- ACE2 receptor: angiotensin converting enzyme 2. This membrane-bound receptor is a protein involved in regulating the renin-angiotensin system, a hormone system important in maintaining blood pressure as well as fluid and salt balance. This receptor appears to be a double-edged sword; in addition to protecting against lethal lung failure in SARS, it has been identified as an essential receptor for SARS coronavirus infections [1,2].
- hrsACE2: human recombinant soluble ACE2. The ACE2 receptor, without the portion that binds to the cell membrane, can be produced on a large scale in the laboratory. The idea is to use hrsACE2 to bind to and ‘capture’ SARS-CoV-2, meaning the virus will no longer be able to enter the cells of affected individuals.
- Alveoli: tiny balloon-shaped air compartments in the lungs where oxygen is captured from the air and passed on to the bloodstream and carbon dioxide accumulated in the blood is released back to the air.
- Clinical trial: a study performed in human volunteers to determine whether a new treatment for a specific disease is safe and works, if it is more effective than the current treatments and if it has any side effects.
- Acute respiratory distress syndrome (ARDS): severe life-threatening condition caused by inflammation from a lung infection/injury. Such inflammation leads to an accumulation of liquid in the lungs that makes breathing difficult.
- Drug development: the process that a newly identified molecule with potential positive effects in the treatment of a certain disease needs to undergo in order to become an approved pharmaceutical drug.
The key words in bold are explained in the drop-down glossary above.
The COVID-19 pandemic, believed to have originated in Wuhan in December 2019, has impacted almost the entire world, with Europe being the most affected area. An international group of scientists studied hrsACE2, a molecule already in clinical trials, to see if it could efficiently prevent infection by SARS-CoV-2.
The study shows that:
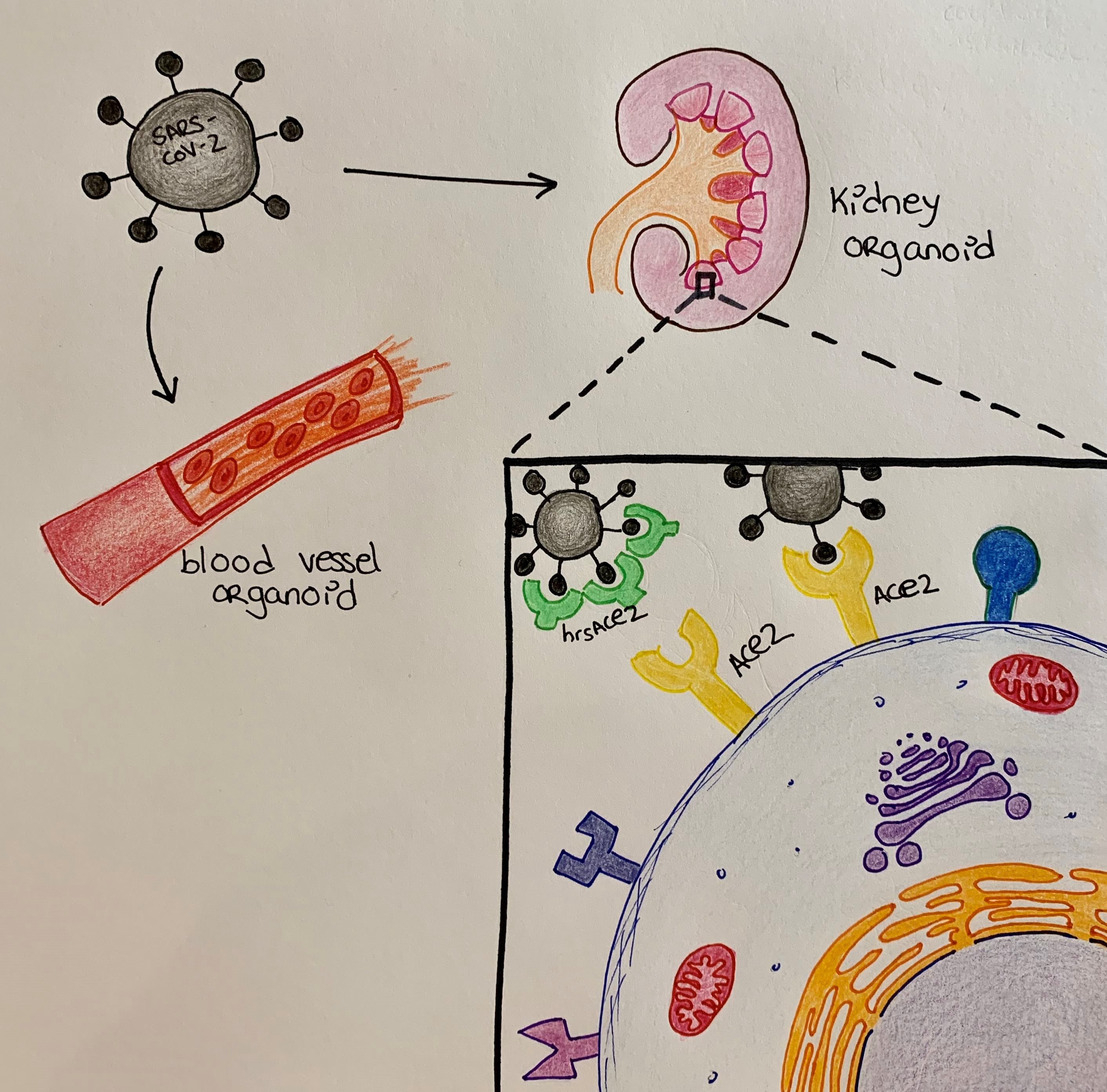
- SARS-CoV-2 can infect and replicate within lab-grown mini human blood vessels and kidneys
- Treatment with hrsACE2 can prevent this infection
- The treatment effectiveness depends on the amount of both hrsACE2 and the virus
The authors conclude that hrsACE2 could potentially help to block SARS-CoV-2 infection, although the study is limited to mini blood vessels and kidneys, and more research is needed to study the effects of hrsACE2 treatment in late stages of COVID-19.
What is the idea behind this study?
The biological agent responsible for the COVID-19 pandemic is a coronavirus named SARS-CoV-2. This virus has been shown to be very similar to the coronavirus (SARS-CoV) that caused the SARS respiratory pandemic in 2002.
One of the shared characteristics between SARS-CoV and SARS-CoV-2 is the mechanism they use to enter human cells, through the ACE2 receptor [3]. The broad distribution of this receptor on the surface of cells throughout the human body, including cells of the alveoli in the lungs as well as specialised cells of the small intestine, kidney, smooth muscle, heart, arteries and veins [4,5,6,7] could explain the extensive variety of symptoms in patients infected by SARS-CoV-2.
This study aims to find a way to prevent the attachment of the virus to the ACE2 receptor on the surface of our cells. More specifically, the authors evaluate the potential of a lab-designed version of this same receptor, which they call human recombinant soluble ACE2 (hrsACE2), to block the entrance of SARS-CoV-2 into human cells. hrsACE2 is already in clinical trials for the treatment of acute respiratory distress syndrome, a disease characterised by breathing difficulties, a symptom also experienced by patients with severe COVID-19 disease. There are two main advantages of using treatments that have already passed some phases of a clinical trial. Firstly, the possibility of the treatment reaching clinics is higher since the safety of the treatment has already been studied. Secondly, because the treatment is more advanced in the drug development process, it could be approved for use within a shorter time frame.
What did this study show?
The authors first isolated the SARS-CoV-2 virus from a patient swab and made multiple copies of it. This allowed them to briefly characterise the virus’s shape and genetic material.
After this initial characterisation, the researchers tested whether the hrsACE2 receptor could block the entrance of the virus into mammalian cells. To do that, they first used a simple cell culture method (monkey cells grown in a dish) to test different amounts of virus and hrsACE2. From this first set of experiments they concluded that:
- The presence of hrsACE2 can inhibit the entrance of the virus into cells grown in the lab
- The inhibitory effect depends on the initial amount of both virus and hrsACE2
- The treatment with hrsACE2 does not have a toxic effect on the cells
The authors next used two much more complex 3D cellular systems, namely blood vessel and kidney human organoids, to test viral infection and the effect of hrsACE2. First, from day 3 to 6 of infection, they detected increasing amounts of viral RNA, indicating the capacity of the virus to copy its genetic material within human organoids. Next, by transferring the nutrient-containing liquid where virus-infected organoids had been grown to monkey cells kept in a dish, the authors show that these cells become infected. Therefore, they conclude that SARS-CoV-2 can infect human organoids as well as generate more infectious viral particles within those organoids. Last but not least, the authors showed that treatment with hrsACE2 could block the entrance of SARS-CoV-2 into the lab-grown mini-blood vessels and kidneys. These results indicate the potential of hrsACE2 to slow down and/or prevent SARS-CoV-2 infection.
What does this mean for patients?
Altogether, the results of this study seem to indicate that hrsACE2 could potentially be used to block the entrance of SARS-CoV-2 into the cells of our body. So far, treatment of COVID-19 is based on the use of general antiviral drugs or on alleviating symptoms, however there is not yet a treatment directly tackling the SARS-CoV-2 virus itself. Notably, the approach taken in the discussed study is actually aiming to interfere directly with the virus by preventing its ability to enter, and thus infect, the cells in our body.
However, the authors also forewarn that the effects of hrsACE2 at later stages of infection are unknown. Hypothetically, one could imagine that, once a person has been infected, with the virus replicating in their cells and released to infect other cells, hrsACE2 may be able to slow down the progression of the infection. This should therefore be studied before using hrsACE2 as a treatment for COVID-19.
To assess how hrsACE2 could potentially contribute to COVID-19 treatment, it is also important to better understand the disease itself. It is known that most people (about 80% according to WHO) recover without needing specialist treatment. The likelihood of becoming seriously ill with COVID-19 is related to previous medical conditions such as heart disease, diabetes or high blood pressure (WHO). Furthermore, patients with serious illness generally experience breathing difficulties. In this context, the authors acknowledge that they should expand their studies to include lung and heart organoids, two tissues highly affected by SARS-CoV-2 and known to express the ACE2 receptor.
Original paper:
Monteil et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell, 2020.
https://www.sciencedirect.com/science/article/pii/S0092867420303998?via%3Dihub
The efficient blocking effect demonstrated in organoids suggests hrsACE2 is a good candidate for clinical trials. Because the drug has already undergone phase I and II clinical trial testing for lung disease, a phase II trial has been initiated by Apeiron Biologics.
These are promising steps, but it is important to reiterate there is currently no approved treatment for COVID-19, and there is much still to be understood about the disease. This is illustrated by an ongoing debate about ACE2 inhibitors, related to the link between previous medical conditions and an increased likelihood of developing COVID-19 complications. A leading general medical journal, The Lancet, published a correspondence (not a research study) hypothesising that, given patients with high blood pressure or diabetes, both conditions treated with ACE2 inhibitors, present higher numbers of ACE2 receptors, the use of ACE2 inhibitors might lead to an increased risk of COVID-19 [8]. However, this hypothesis has been challenged and the discussion is ongoing in the field. At the time of writing, health agencies and professional organisations, including the European Medicines Agency and the European Society of Cardiology, have said there is insufficient evidence to change existing treatment plans for these conditions [9,10].
It is important to note that hrsACE2 is not an ACE2 inhibitor, but this controversy highlights that, while many scientists are working tirelessly to understand COVID-19 and identify ways to treat it, this is not an easy task, nor a fast process. It is important to go through rigorous clinical trials with large numbers of patients to identify possible adverse effects and ensure the effectiveness of new treatments.
Original paper:
Monteil et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell, 2020.
https://www.sciencedirect.com/science/article/pii/S0092867420303998?via%3Dihub
Other references:
- Kuba et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature, 2005. https://www.nature.com/articles/nm1267
- Kuba et al. Trilogy of ACE2: A peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacology and therapeutics, 2010. https://www.sciencedirect.com/science/article/pii/S0163725810001415?via%3Dihub#bb0315
- Zhou et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 2020. https://www.nature.com/articles/s41586-020-2012-7
- Hamming et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 2004. https://onlinelibrary.wiley.com/doi/full/10.1002/path.1570
- Donoghue et al. A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circulation Research, 2000. https://www.ahajournals.org/doi/10.1161/01.RES.87.5.e1
- Tipnis et al. A Human Homolog of Angiotensin-converting Enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. Journal of Biological Chemistry, 2000. https://www.jbc.org/content/275/43/33238
- Wadman et al. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Science, 2020. https://www.sciencemag.org/news/2020/04/how-does-coronavirus-kill-clinicians-trace-ferocious-rampage-through-body-brain-toes?utm_campaign=news_weekly_2020-04-17&et_rid=552722233&et_cid=3290457
- Fang et al. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet – Respiratory Medicine, 2020. https://www.sciencedirect.com/science/article/pii/S2213260020301168?via%3Dihub
- EMA advises continued use of medicines for hypertension, heart or kidney disease during COVID-19 pandemic, Press release 27/03/2020. https://www.ema.europa.eu/en/news/ema-advises-continued-use-medicines-hypertension-heart-kidney-disease-during-covid-19-pandemic
- Position Statement of the European Society of Cardiology Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers, 13/03/2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
Written by Sílvia Llonch. Reviewed by Kirsty Ferguson. Edited by Amanda Waite.
Drawing by Sílvia Llonch.
Infected blood vessel organoid image reproduced with kind permission from IMBA. Copyright IMBA/Kulcsar.
Zuletzt aktualisiert: