Treating Huntington’s disease: making new neurons is not enough
Many researchers and clinicians believe that stem cells will one day be used in regenerative medicines to treat many injuries and diseases, including Huntington’s disease (HD). Researchers think that nerve cells that die in HD patients may be able to be replaced with new healthy neurons made from stem cells. Scientists are already able to use stem cells to create nerve cells similar to those lost by HD patients. However, researchers from Cardiff University say that repairing a HD patient’s brain will likely require more than just replacing one type of nerve cell. Reddington and colleagues present evidence in their 2014 review in the journal Frontiers in Cellular Neuroscience indicating that treating a HD patient will necessitate implanting many types of cells. These various cells, in the right combination, will most likely needed to successfully incorporate the new neurons with neurons still in the brain of a HD patient.
What is the idea behind this review?
Huntington’s disease (HD) is a genetically inheritable disease passed on from parents to their children through a specific gene. This HD gene instructs cells to make an abnormal form of a protein called ‘huntingtin’. Neurons, the cells that send signals throughout our brain, need the huntingtin protein to stay healthy, but the abnormal form of huntingtin causes many problems and becomes toxic to neurons. The neurons most sensitive to the abnormal form of the huntingtin protein are found in a region of the brain called the ‘striatum’.
The ‘striatum’ is a region of the brain with many functions. It controls our motivation, emotional responses, personal habits and coordinating our voluntary movements. The striatum is made up of many different cells and neurons, including ‘medium spiny neurons’. It is the medium spiny neurons that are most sensitive to the abnormal form of huntingtin protein. As medium spiny neurons die, HD patients lose motivation and become apathetic, develop jerky movements or stiff limbs, and may lose emotional attachment towards people and things they previously valued.
Although there is presently no treatment for HD, researchers are hopeful that future regenerative medicine treatments may be possible. Many researchers hope to create treatments to replace destroyed medium spiny neurons in HD patients by transplanting cells that can take over their function. One important constraint is that the donor cells have to be immature ones that are still undergoing development, and to date this has necessitated using cells collected from foetuses. Researchers would like to generate immature medium spiny neurons from pluripotent stem cells, cells that can create any type of cell found in the body. Researchers have already successfully made cells similar to medium spiny neurons from stem cells in the laboratory. So the question is: will implanting these cells into the brains of HD patients be a successful treatment for HD?
What insights did the review present?
Reddington and colleagues describe numerous aspects of biology that scientists and clinicians have learned about while researching the transplantation of neurons into the brain. Researchers now know much more about how brain cells change during different stages of brain development and which of these developmental stages has cells that can be successfully transplanted. Transplanting neurons has been done successfully in rodents, primates and even humans. Clinical trials in HD patients showed that transplanted neurons successfully integrate with neurons already present in the striatum. Although transplants in HD patients did not prevent long-term progression of HD disease, patients did show improvement and some lessening of typical HD symptoms approximately 18 months after treatment. Some patients continued to show functional recovery six years after treatment. These studies suggest there is hope of creating an effective and reliable treatment for HD. However, there are complications. One of which is that all these studies, including the clinical trials described above, involve transplanting brain cells taken from human foetuses.
Many researchers want to avoid the moral and ethical issues of taking cells from a foetus. Aside from the ethical issues, there are also many technical reasons why using cells from a foetus is not a viable method for treating HD patients (such as quality control and the number of cells needed for a single treatment). This is why many researchers are attempting to use pluripotent stem cells to make the ‘medium spiny neurons’ that HD patients need. Particular stem cells, called ‘induced pluripotent stem cells,’ are created from cells taken from a patient, such as skin cells. This helps to avoid some moral issues. Researchers have tried using stem-cell-derived neurons, but many of these studies show that stem-cell-derived neurons do not integrate into the existing brain cells as well as cells transplanted from a foetus brain do.
Reddington and colleagues propose that the reason neurons made from stem cells are not successful in transplants is because researchers have been trying to transplant just one type of neuron, medium spiny neurons. Transplants from foetal tissue contain many different types of cells, including medium spiny neurons and another type of neuron called ‘interneurons’. Reddington and colleagues suggest that if stem cells are to be successfully used, researchers will need to make a larger diversity of cells to be implanted. Additionally, this will require knowing the correct mixture of the different types of cells. The bottom line is: using stem cells to make only new medium spiny neurons for implanting may not be enough to treat HD patients.
What does this mean for people affected by Huntingdon's Disease?
The good news: Researchers have learned enough to create cells similar to medium spiny neurons from stem cells. Researchers now also understand much more about the biology that naturally builds our brains during development. The more researchers know, the more they can use that knowledge to harness and implement natural ability of our cells to grow and heal our body. This information has already helped researchers to transplant neurons from one brain to another. Although these transplants are still not perfect, it’s still quite amazing that neuron transplants are possible in humans. These encouraging results are hopefully just the beginning. Collaborations and research groups such as the European Union’s Repair-HD Project and NeuroStemCell Repair Consortium will hopefully continue to speed up and support the research needed to attain an effective treatment for HD patients.
The not-so-good news: A viable treatment for HD still looks to be a long time off. Using stem cells to treat HD disease is going to be complicated because a range of cells are likely required for successful brain cell transplants in patients. Stem cells offer numerous advantages and avoid many ethical issues, but they also have their own complications. Researchers first must learn how to control stem cells to reliably make specific types of neurons and other cells. This takes years and lots of work. Once a procedure is known, it still takes weeks or even months to make some types of cells. It’s lots of labour, time and money.
Although there are a huge number of issues to overcome to make a treatment possible for HD disease, remember that science is quickly moving forward. Yes, there’s lots of work to be done, but discoveries are made every day and research is getting faster. Ultimately, it’s just a matter of time.
Further information and links
HDBuzz is a well written website that has summaries research articles about Huntingdon's Disease written in plain language.
This summary is based on the original article ‘Differentiation of pluripotent stem cells into striatal projection neurons: a pure MSN fate may not be sufficient’ by Amy E. Reddington et al., Frontiers in Cellular Neuroscience 2014. A journal subscription may be required for access.
A great source of on-going information on using pluripotent stem cells to treat Huntington’s disease can be found at Repair-HD. This website provides the public information on a four-year collaborative research project funded by the European Union’s Seventh Framework Programme for research. Another research funded project focused on using stem cells to repair the human brain is the NeuroStemCell Repair Consortium.
Wikipedia offers a description of the huntingtin gene and protein mentioned in this article. Please note that these are rather technical descriptions.
Acknowledgements
Written by Dr. Ryan Lewis, edited by Dr. Jan Barfoot, reviewed by Dr. Anne Rosser.
-----
FIGURE LEGENDS
Image of the brain / striatum
The ‘striatum’ is a part of the brain important for associating emotions with people and things around us, it is shown in red in the image above. Two examples of this would be knowing that we love a person when we see his or her face and recalling memories and emotions associated with a specific smell or song. The striatum is also important for coordinating signals to our muscles that allow us to make smooth and controlled movements.
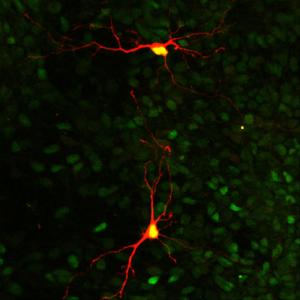
Image of striatal medium spiny projection neurons
Symptoms associated with Huntington’s disease are caused by the death of neurons in a patient’s brain. ‘Medium spiny neurons’, shown here in orange, are the primary type of neurons that die from the disease. Medium spiny neurons are found in the part of the brain called the ‘striatum’, which is important for emotions, motivation and movement.