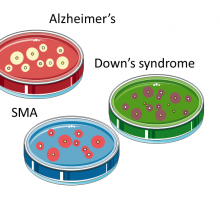
EuroStemCell is here to help European citizens make sense of stem cells.
As a network of scientists and academics, we provide independent, expert-reviewed information and road-tested educational resources on stem cells and their impact on society. We also work with people affected by conditions, educators, regulators, media, healthcare professionals and policymakers to foster engagement and develop material that meets their needs.
EuroStemCell Resources

All about Stem Cells: Flexible Stem Cell Teaching Tool
• EuroStemCell resource
Stroke: how could stem cells help?
• EuroStemCell resource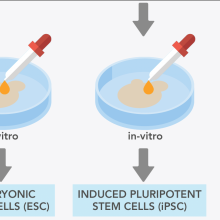
Reproduction and Fertility: How could stem cells help?
• EuroStemCell resource