Diabetes: how could stem cells help?
Diabetes is a common life-long condition and the number of children being diagnosed with type 1 diabetes is increasing. The symptoms can be controlled but there is no cure. For many, diabetes means living with daily insulin injections and the possibility of long-term damage to their health.
When blood glucose (sugar) levels rise, beta cells in the pancreas release insulin. Insulin tells cells throughout the body to take up glucose from the blood. Glucose is the most important source of energy for all cells in the body.
In Type 1 diabetes, the immune system destroys beta cells. In Type 2 diabetes, cells do not take up enough glucose, either because they are insensitive to insulin or too little insulin is produced.
Type 1 diabetes patients require daily blood testing and insulin shots.
Scientists have successfully used pluripotent stem cells to produce glucose-responding cells that release insulin, like beta cells. Clinical trials of these cells are underway.
Diabetes is quite well understood, but the causes of diabetes are not. Research is still being conducted on what triggers the immune system to destroy beta cells in Type 1 diabetes.
Current research is examining the use of pluripotent stem cells as a way to create beta cells that can be transplanted into patients with Type 1 diabetes. Clinical trials are presently taking place with devices/capsules that protect transplanted stem cell-derived precursor cells of beta cells from the patient’s immune system.
Researchers are also interested in the possibility of using drugs to promote cells in a patient’s pancreas to naturally make more beta cells.
Autoimmunity is a big challenge for Type 1 diabetes. Even if new beta cells are created or transplanted into a patient, the immune system will eventually target and destroy these cells. Thus, treatments must consider how to prevent new beta cells from being targeted. Typically this has involved immune suppressants, which have an unfortunate side effect of increasing the risk of infection.
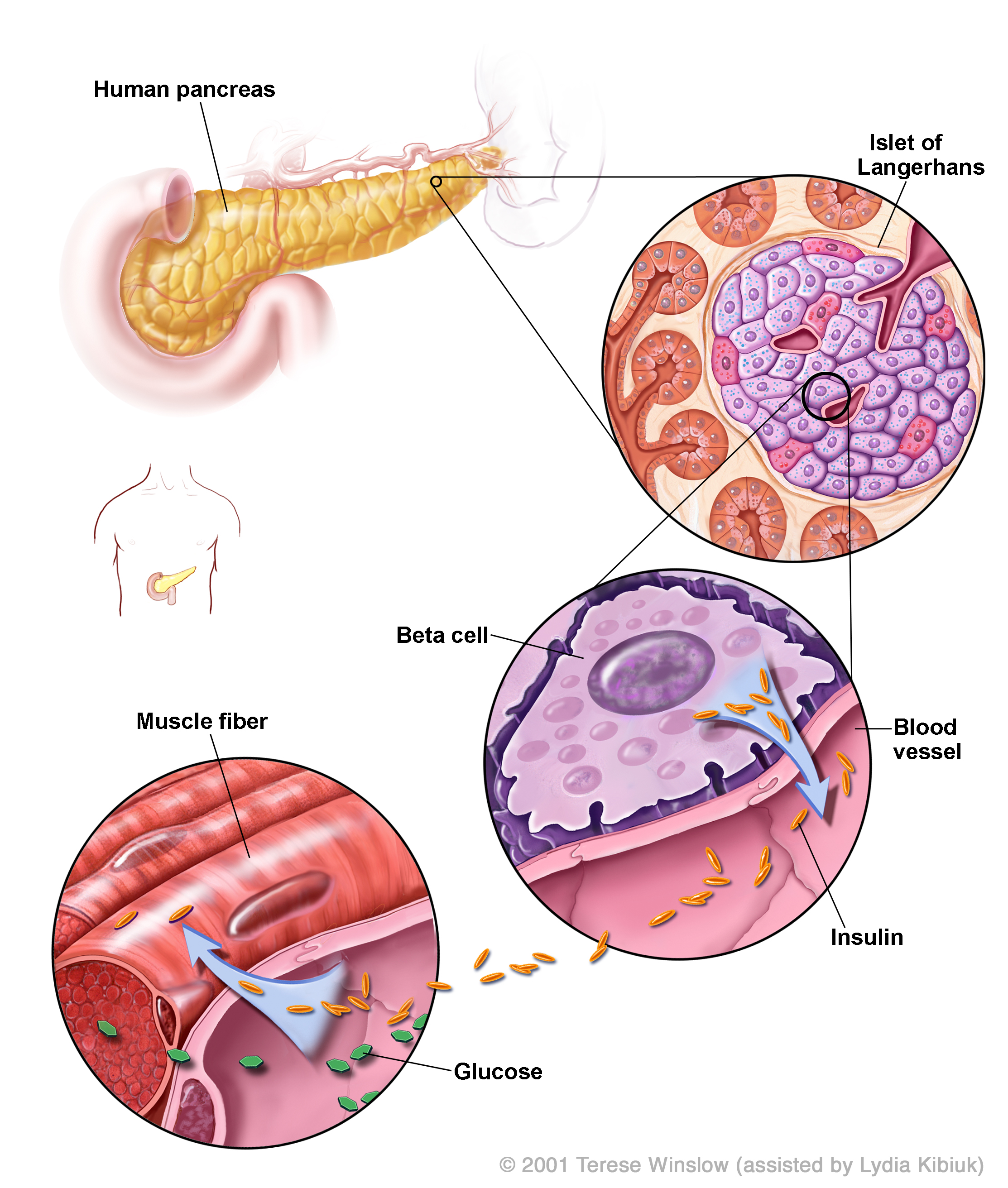
All the cells in your body need energy. This energy is carried around the body as sugar (glucose) in the blood. There are several types of diabetes. What they all have in common is a problem with regulating normal levels of sugar in the blood. Normally, blood sugar levels are controlled by the release of the hormone insulin. Insulin instructs the cells in the body to take up glucose so they can use it as energy. Insulin is made by cells in the pancreas called beta cells that are arranged into clusters together with other pancreas cells. These clusters are called islets of Langerhans. In one human pancreas there are roughly one million islets.
Where is the pancreas?: located in the abdomen, next to the small intestine and stomach. The cells in the pancreas that make insulin (beta cells) are highlighted in red in this video by Dror Sever and Anne Grapin-Botton.
There are several types of diabetes. What they all have in common is a problem with regulating normal levels of sugar in the blood.
The most common types of diabetes:
Type 1 diabetes occurs when the body’s immune system damages and then destroys beta cells. As a result, no insulin is being produced. This means the levels of sugar in the blood stay high all the time, which can lead to long-term damage to the body.
Type 2 diabetes occurs when insulin no longer works correctly as a signal to the body's cells that sugar should be absorbed. This might be because cells are unresponsive to insulin, or that beta cells are producing too much or too little insulin, or a combination of both of these cases.
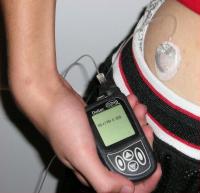
Currently there is no cure for diabetes. Although Type 2 diabetes can often be at least partially controlled by a healthy diet and regular exercise, Type 1 diabetes cannot. People with Type 1 diabetes must self administer insulin to survive (through injections or a pump) and test their blood sugar levels several times a day in order to determine insulin dosages. Unfortunately, it is difficult to keep the blood sugar level within a normal range. Over time, high blood sugar levels can cause serious damage to the heart, eyes, blood vessels, kidneys and nerves, whilst injecting too much insulin can lead to a blood sugar level that is too low (hypoglycaemia) which can be fatal.
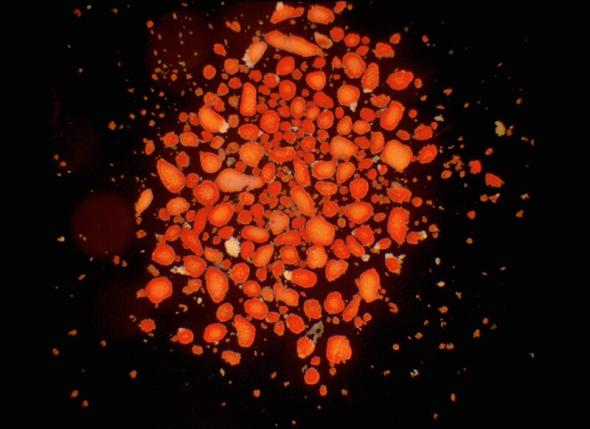
It is possible to treat Type 1 diabetes by transplanting pancreatic islets, isolated from a donor pancreas, or even a whole donor pancreas into the patient. Transplants can enable the body to regain control of blood sugar levels so that administrating insulin is no longer needed. A whole pancreas transplant involves major surgery and carries significant risk. Islet transplantation is usually performed by an infusion of isolated donor islets into the portal vein of the liver, which becomes the home of the new islets. Transplantations are dependent on available donors.
There are problems with islet transplantations:
- The number of donors is heavily outweighed by the demand and the islets have to be of good enough quality and in the right amounts.
- Transplantation of donor cells (whole pancreas or islets) imply that the immune system needs to be suppressed so that the transplanted donor pancreas or islets are not rejected. The immune suppressing drugs leave the recipient vulnerable to infection and often have side-effects. Today only a limited number of patients with type 1 diabetes are suited for transplantation due to these side effects.
Even with immunosuppressive drugs there is a gradual decline in transplant function, which is likely due to chronic rejection and further transplants may be needed. If the immune system has been triggered to destroy donor cells, it may be more difficult to find an appropriate second transplant as further islet and other transplants like a kidney are more likely to be rejected.
There are currently no proven treatments for diabetes using stem cells. If beta cells currently made in the lab from pluripotent stem cells can be proven to be functional enough in the current ongoing clinical trials, it could solve the problem of obtaining the right number of functional islets for future transplantation needs.
Current approaches to make new beta cells for therapy:
- Mature beta cells derived from human pluripotent stem cells in the lab, which are transplanted into diabetic patients.
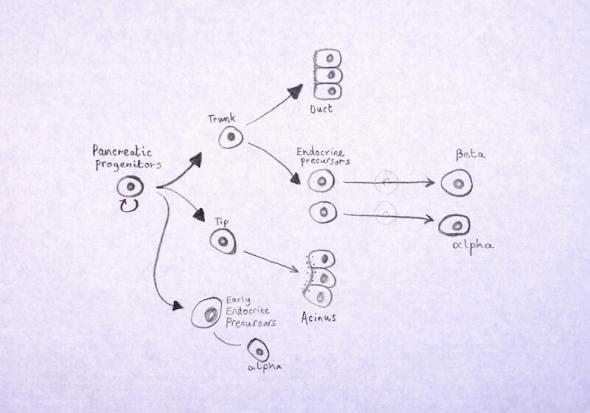
- Mature human pluripotent stem cells into pancreatic progenitor cells in the lab, which are transplanted into diabetic patients where they will mature into functional beta cells.
- Mature beta cells in the lab from other types of cells, for example liver cells, which are transplanted into diabetic patients.
- Use drugs to trigger cells in the diabetic patient’s own pancreas to produce new beta cells.
- Islet-like clusters consisting of both alpha and beta cells derived from human pluripotent stem cells. This is currently being explored in the EU-funded project ISLET.
And, for all of these approaches ongoing research is exploring:
- How can we protect the cells from being attacked by the immune system once they have been transplanted?
Making beta cells from pluripotent stem cells
Pluripotent cells (either embryonic stem cells or induced pluripotent stem cells) can make any cell type in the body and researchers are exploring how to direct these to make fully functional beta cells. Such cells could replace the scarce source of donor pancreatic islets of Langerhans. Researchers have so far succeeded in producing cells from human pluripotent stem cells that respond to glucose in a similar way to normal beta cells both in the laboratory and in mice. Moreover, when transplanted in diabetic mice glucose levels become normalized. These beta cells will soon be tested for safety in phase 1 clinical trials.
Making beta cells from other cells
Some researchers think it might be possible to encourage cells already present in the patient’s pancreas to make new beta cells – a process normally referred to as regeneration. It is not known whether stem cells exist in the pancreas but beta cell precursors have been found in mice. Some researchers hope that if these precursors exist in the human pancreas that they may be able to find drugs that convert them into new beta cells in patients. Obviously, even if this would be possible one would also have to prevent the autoimmune destruction of the new-born beta cells. These efforts are still experimental in nature and have not reached a point where clinical trials are close.
Making islet-like clusters from pluripotent stem cells
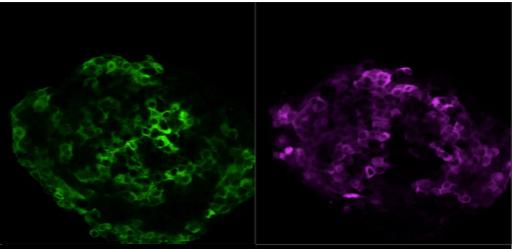
Human islet transplantation studies have shown that human islets consisting of both alpha cells and beta cells have superior glucose-stimulated insulin secretion and metabolic function compared to clusters consisting of only beta cells. Beta cells secrete insulin in response to glucose when glucose levels are too high. Opposite, when glucose levels are too low, glucagon is being secreted from alpha-cells to direct the release of glucose from the liver into the blood-stream. When the two cell types work together in a therapy, they will create a finer tuned response to glucose levels in the blood.
Therefore, islet-like clusters consisting of a mixture of beta-cells and alpha cells could be superior to only transplanting beta-cells into the patients. As mentioned previously in the text, beta-cells can be generated from human pluripotent stem cells. Likewise, using similar differentiation protocols, alpha cells can be generated from pluripotent stem cells.
This is the scope of the European funded ISLET project. You can find more information here: https://isletproject.eu/
Protecting cells from the immune system

Work is underway to find the most effective way of encapsulating transplanted cells to protect them from immune attack. At the moment several research groups and commercial companies (ViaCyte and Beta-O2Technologies) are involved in clinical phase 1 studies to create a capsule that allows for inward transport of glucose and other nutrients and outward transport of insulin yet protecting the cells from the immune system.
Viacyte are currently testing two devices:
PEC-Direct (VC-02): blood vessels can enter the device and vascularize the implanted cells. Immunosuppressants are still needed.
PEC-Encap (VC-01): implanted cells are contained in the device. Oxygen, glucose and insulin and other hormones can travel between the implanted cells and the blood vessels outside of the device. The implanted cells are protected from the immune system in this device.
Beta-O2 Technologies is based on a hydrogel which functions as a scaffold for encapsulation of the cells.
Work exploring drugs, vaccines or cells that reduce the immune reaction is also underway. Results of this work could create better therapies by being combined with beta cell transplantation.
Transplanting progenitor cells made from pluripotent cells
The fact that it is possible to efficiently generate beta cells from pluripotent stem cells strongly argues that it is time to test the safety and efficacy of these cells in patients with Type 1 diabetes. Two clinical trials for type 1 diabetes involving stem cells have been launched in recent years. Both run by biotech company ViaCyte, they involve the use of pancreatic precursor cells that are placed in a credit-card-like case and transplanted into the body. The hope is that, similar to studies in mice, the precursor cells will spontaneously mature into insulin-producing cells in the body and replace the lost beta cells.
The first clinical trial was started in July 2014. This Phase 1/2 clinical trial aimed to investigate safety and efficacy of the PEC-EncapTM product, which consists of precursor cells loaded in a device that allows the release of insulin into the blood stream whilst preventing the immune system from attacking the cells. ViaCyte reported in 2018 that PEC-Encap is safe and well-tolerated by patients, with the Encaptra® device appearing to be immuno-protective as designed. However, further development to ensure effective engraftment in patients is needed. Towards this end, ViaCyte is collaborating with W. L. Gore & Associates to generate new devices for implantation.
In 2017, ViaCyte initiated another Phase 1/2 trial in USA and Canada, in which the same precursor cells are transplanted in a device that allows direct vascularization of the cells (VC-02). Because this device requires immunosuppression to avoid graft rejection, it is meant to be used in patients with type 1 diabetes that are at high risk for acute complications, including coma and death. Thanks to a long-standing collaboration with the international Beta Cell Therapy consortium (www.betacelltherapy.org/), a new arm launched in November 2018 with the implantation of the first European patients in Brussels, Belgium.
Diabetes Atlas - statistics from the International Diabetes Federation (IDF)
International Diabetes Federation
ISLET - A European project innovating advanced cell-therapy for Diabetes
Innovative Medicines Initiative for Diabetes (IMIDIA)
Diabetes UK - information and patient support
My Life - information for younger people living with diabetes
Centre for beta cell therapy - research consortium
ViaCyte – information about the first human clinical trial for diabetes
Classroom resource about stem cells and diabetes using real science data
This factsheet was first created by Sarah Pattison.
Reviewed in 2011 by Harry Heimberg.
Reviewed and updated in 2015 by Johan Olerud, Cathy Southworth and Henrik Semb.
Reviewed and updated in 2018 by Henrik Semb and Eelco de Koning.
Reviewed and updated in 2020 by Maya Kjærgaard.
Images and videos
(copyright resides with the named contributor unless otherwise noted)
- Human islet where beta and alpha cells are highlighted in green and violet, respectively, using insulin and glucagon antibodies © Olle Korsgren.
- Healthy and type 1 diabetes islet images © Johan Olerud
- ‘The pancreas’ video showing the pancreas, stomach and Duodenem © Dror Sever and Anne Grapin-Botton
- 'Insulin Production in the Human Pancreas' diagram © 2001 Terese Winslow (assisted by Lydia Kibiuk).
- Diabetes injection image © Wellcome Library, London.
- Insulin producing cells from embryonic stem cells: C-peptide staining of insulin-producing cells derived from hESCs courtesy Katja Hess/Zarah Löf Öhlin
- ‘Isolated human islets of Langerhans used for transplantation’ by Andrew Friberg (CC BY 3.0)
- The birth of beta cells by Cameron Duguid (CC BY 3.0)
- Capsule for transplanting cells © ViaCyte